UID, or Unique Identification, is the process of marking an item with a globally unique identifier and ensuring data integrity throughout the item's life cycle.
UID marking ensures data integrity and data quality throughout life; it also supports multi-faceted business applications by enhanced total asset visibility, improved lifecycle item management and accountability, hyper-managed recall and clean financial audits.

I-GUIDES® may be considered as your organization's own
item-level registry
in a highly accessible, web-based, flexible and secure platform.
I-GUIDES® is easily configured to work in any industry and with any program or protocol whether conforming or proprietary.
Below are several UID standards that I-GUIDES® supports. I-GUIDES® is not limited to these standards.
IUID - Item Unique Identification (Government / Defense)
IUID is a U.S Government mandate implemented by the Department of Defense. A permanent marking method is used to assign assets (items) a unique ID. The U.S. DoD made IUID marking required for all equipment with an acquisition cost of over $5,000, equipment which is mission essential, controlled inventory, serially-controlled or consumable.
The Office of the Secretary of Defense (OSD), in a policy statement released July 29, 2003, mandated unique marking and registry (UID) of specific items covered in DFAR Case 2003-D081. This Policy makes UID a mandatory DoD requirement on all solicitations issued on or after January 1, 2004.
The current rule can be downloaded here: MIL-STD-130M

An item will be uniquely identified if:
- the acquisition cost is $5,000 or more
- it is either a serially managed, mission essential or controlled inventory piece of equipment or a reparable item, or a consumable item or material where permanent identification is required
- it is a component of a delivered item, if the program manager has determined that unique identification is required,
- a UID or a DoD-recognized UID equivalent is available.
STANAG 2290 (NATO)
STANAG 2290 is the guidance for NATO nations to an established UID standard. The agreement provides instruction on UID creation and asset marking. This "common language" enables asset management to be leveraged across different MoD organizations. I-GUIDES® is fully compliant to STANAG 2290. Every participating nation is encouraged to create their own policy and registry for their assets. With I-GUIDES®, the internal registry is established out of the box . Additionally, I-GUIDES® quickly and easily implements local rules and policies through its extraordinary comprehensive and patented customizable features.

Further more information, view the STANAG 2290 policy
UDI - Unique Device Identification (Medical)
The UDI system is intended to assign a unique identifier to medical devices within the United States. It was signed into law on September 27, 2007, as part of the Food and Drug Administration Amendments Act of 2007. The UDI is expected to improve patient safety.

GS1 - Item Unique Identification (Retail)
GS1 is an international non-profit association dedicated to the development and implementation of global specifications to management of supply and demand chains across multiple sectors. ISO is the main world wide standardization organisation. GS1 publishes the GS1 General Specifications. These are based fully on ISO standards.

Commercial UID
The industries above are leveraging automatic identification technologies (AIT) to enable UID which, simply stated, assigns a unique pedigree to an item (asset).
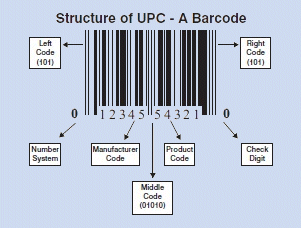
A perfect example of how AIT promotes efficiencies is the retail industry's application of UPC codes to items. This product-level marking began in the 1970s. It established a registry that makes life easier for all of us. UID takes UPC one step further by assigning a globally unique identifier to each item or asset, enabling a higher level of management throughout an item's lifetime.